Lets make an Isotope
The vast majority of isotopes are short-lived and typically not found on our planet. How can scientists learn anything about nuclei that:
-
- Are invisible to the most powerful microscopes
- Do not occur naturally on Earth
- Have half-lives of less than a second, often less than a millisecond
- Produce radiation with their rapid decay
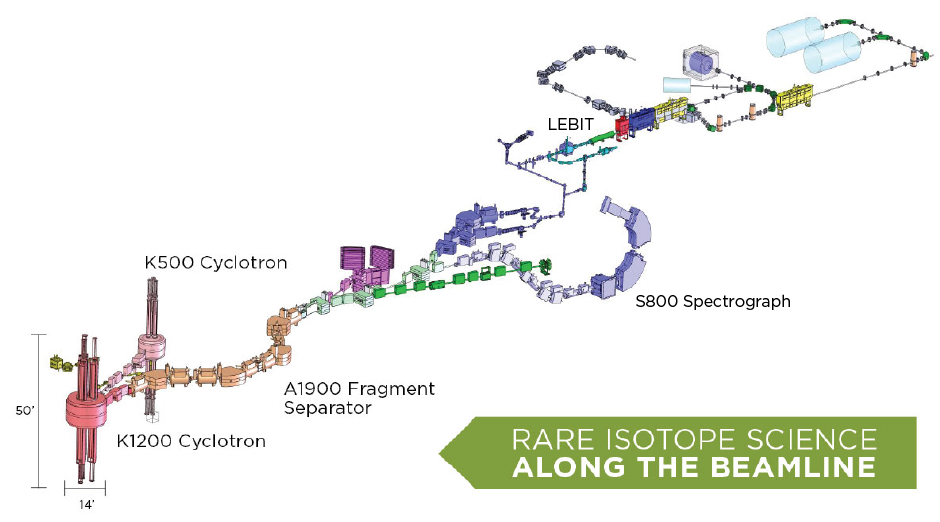
NSCL enables world class discoveries with rare isotope science along the beamline, which begins with two cyclotrons:
-
To study rare isotopes, we must first produce them. Ion sources strip electrons from atoms of a stable, common isotope, then feed the partially ionized atoms to the K500 cyclotron to accelerate them up to 30,000 miles per second or 15 percent of the speed of light. The larger and more powerful K1200 cyclotron boosts the ions up to half the speed of light.
-
The stream of ions then smashes into a thin foil target where the fast nuclei “fragment” (break) through collisions with stationary nuclei in the foil. This creates a wide variety of new, possibly rare and radioactive isotopes. These resulting isotopes are filtered with the A1900 Fragment Separator, which carefully selects particular rare isotopes from the fast beam of nuclei and steers them through.
-
Now that a rare isotope has been made, NSCL detectors can measure properties of these nuclei very quickly. One example is the three-story, 300-ton S800 Spectrograph, located at one endpoint of the beamline. This device can rotate 150º to detect particles released after rare isotope nuclei smash into another target. The S800 measures the structure (which includes size and shape) of the nucleus.
-
Another detector, the Low Energy Beam Ion Trap (LEBIT) actually weighs extremely short-lived isotopes by trapping them in a powerful magnetic field.



